The National Institute for Occupational Safety and Health (NIOSH) developed USP General Chapter <800> to better protect all workers, patients and general public who may potentially come in contact with hazardous drugs. Many hospitals are keeping this top of mind as they try to understand how these requirements impact their pharmacy operations. If your hospital pharmacy currently repackages hazardous drugs in-house, you may need to implement new steps and process changes to be compliant with USP <800> (official December 2019).
We understand that changes like this can be challenging. We’ve put together a brief overview of the hazardous drug labeling, packaging and disposal processes you need to put in place to ensure compliance.
What’s considered hazardous?
 NIOSH considers a drug to be hazardous if it exhibits at least one of the following criteria: carcinogenicity, teratogenicity or developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity, or structure and toxicity profiles of new drugs that mimic existing hazardous drugs.
NIOSH considers a drug to be hazardous if it exhibits at least one of the following criteria: carcinogenicity, teratogenicity or developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity, or structure and toxicity profiles of new drugs that mimic existing hazardous drugs.
Any hospital handling these hazardous drugs are required to incorporate USP <800> standards into their occupational safety plan. At a minimum, each health and safety management system need to include a list of hazardous drugs, facility and engineering controls, safe work practices, proper use of appropriate protective equipment and set policies for waste segregation and disposal.
Packaging and Labeling Hazardous Drugs
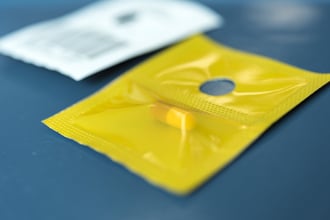
It’s imperative to make sure that all the drugs on the NIOSH list and any additional drugs designated as hazardous by your hospital are properly packaged and labeled — including any repackaged medications. With USP <800>, hospitals are required to clearly denote any drug as hazardous and clearly label any drug handling precautions. Using packaging that very clearly separates hazardous drugs — like Safecor Health’s bright yellow packaging option — can also add another layer of protection.
Hospitals must also ensure that any packaging containers and materials being used for hazardous drugs maintain physical integrity, stability and sterility of the hazardous drug.
Disposing of Hazardous Drugs
To ensure protection of both staff and the environment, any employees performing routine waste removal and cleaning in a hazardous drug area must be trained in appropriate procedures. Your hospital will need to make sure these procedures comply with all applicable federal, state and local regulations.
Avoiding Cross-Contamination
When it comes to packaging hazardous drugs, one of the biggest areas of concern is ensuring that all reusable equipment is properly decontaminated, cleaned and disinfected. With USP <800>, proper written procedures are required to avoid cross-contamination and any personnel performing these tasks must be properly trained. Proper cleaning agents must be used and documented in your cleaning procedures.
Ensuring USP General Chapter <800> Compliance
The best way to ensure USP General Chapter <800> compliance is to simply take the time to read and understand its components, determine what policies and procedures you need to update, and create a plan of action. But don’t be overwhelmed. Keep in mind that some areas can be outsourced to help alleviate stress and costs — like unit-dose packaging with Safecor Health. Partnering with an FDA-regulated, cGMP compliant unit-dose packager can help reduce workloads and facilitate a faster path to compliance.
We’re here to help. Contact us to learn how Safecor Health can help you with USP General Chapter <800> compliance. Need hazardous drugs repackaged today? Get the list of hazardous drugs Safecor Health currently repackages.



